VTT leads €32 million project on photonics for healthcare

The PhotonMed project brings together 39 partners in 9 countries aiming to reduce the time to market for integrated photonics products that could enable faster, more accurate, less invasive, and more personalised medical care
VTT Technical Research Centre of Finland is overseeing the coordination of a project called PhotonMed, which has been provided with €32 million from the Chips JU/EU and national funding agencies. With 39 partners in 9 countries, PhotonMed aims to accelerate the uptake of research innovations in photonics-based diagnostics, surgical tools and therapeutic applications, by ensuring they are directly implementable in manufacturing.
The project seeks to significantly reduce the time to enter the market for advanced photonics technologies in medical devices, that could enable more accurate, faster, less invasive, and more personalised healthcare solutions and diagnostic and therapeutic tools that improve patient outcomes and overall healthcare efficiency.
“Until now, the gap between research and manufacturing has been a major hurdle in introducing photonics innovations for medical applications,” Jussi Hiltunen, research professor at VTT. “Coupled with regulatory validation processes, the time-to-market takes years, incurring high costs. The PhotonMed project aims to bridge the gap by creating structured industrial supply chains and leveraging a pilot line R&D model to facilitate faster commercialisation.”
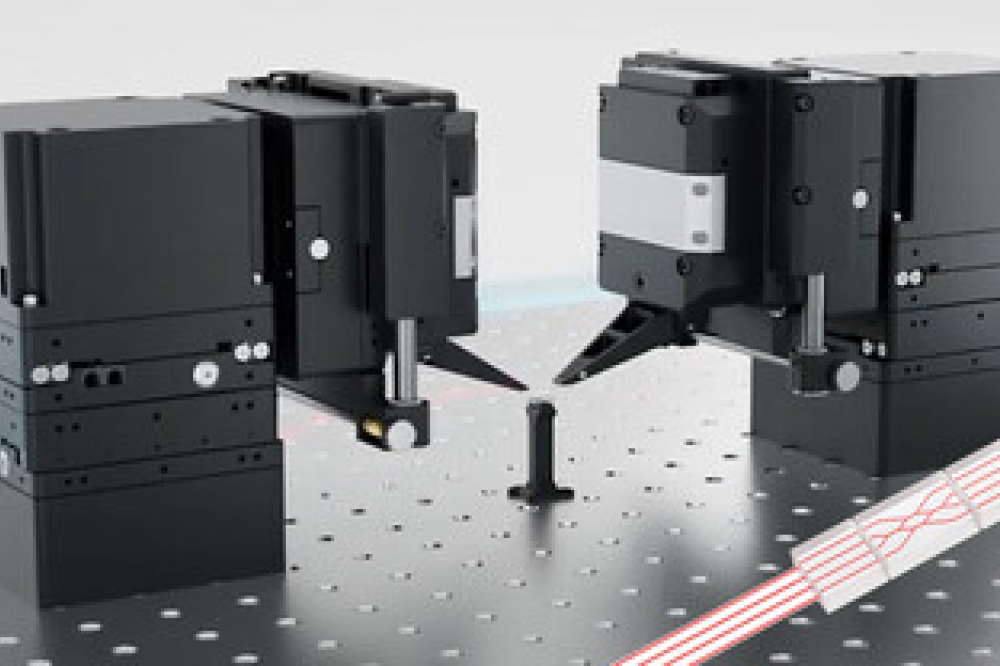
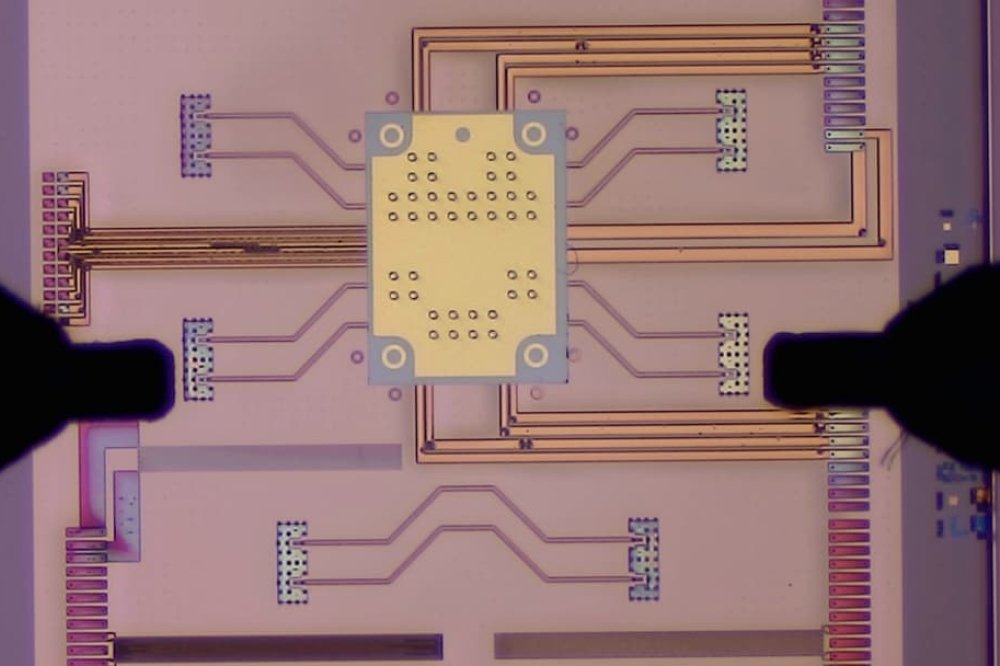
In the PhotonMed project, research is validated through 16 pilot cases led by end-user companies. The photonics technologies applied in these cases include light sources, integrated optics, fibre optics, advanced packaging, microfluidics, and reader instrumentation.
By exploiting pilot line operational model and research infrastructure of various partners, PhotonMed aims at ensuring that new technologies meet stringent regulatory requirements, making it easier for companies to transition from R&D to production.
The PhotonMed consortium consists of 39 partner organisations in 9 countries. It brings together the entire research and supply chain with component manufacturing and end-user companies such as Polar, well-known for its sports watches.
“As a pioneer in wearable technology, we find the PhotonMed project extremely interesting,” says Ismo Savikoski, vice president of Research and Development at Polar. “We are enthusiastically expecting what kind of photonics research results could be introduced as new features in our sports and fitness watches and wellbeing solutions.”
One of the challenges PhotonMed aims to tackle is the need for miniaturisation in developing highly integrated photonic devices. Manufacturing partners such as German-based FiconTEC share this goal.
“FiconTEC is proud to join the PhotonMed consortium, aiming to enhance our integration and production technologies in the biomedical sector while significantly advancing our capabilities to miniaturise and package cutting-edge solutions for the medical device industry,” says Moritz Seyfried, director of R&D at FiconTEC. “We expect to develop and implement automated production methods that will streamline the complex assembly processes of photonics-related medical devices, setting new standards for efficiency and reliability.”
Another expectation is that PhotonMed will facilitate the development and manufacturing processes of components.
Arthur Blom, COO of Surfix Diagnostics, notes: “PhotonMed will support Surfix in developing selected components and processes, thereby increasing our technology and manufacturing readiness levels. Furthermore, PhotonMed will accelerate the establishment of a mature European industrial photonics ecosystem. All in all, we expect that PhotonMed will help us achieve a successful product launch and facilitate future product developments.”