Improved on-chip optical tweezers

MIT researchers have developed a silicon photonics chip that uses light to manipulate particles at a greater distance than existing chip-based optical tweezers, offering advantages for biological experiments
MIT researchers say they have developed miniaturised, chip-based optical tweezers, that could one day help biologists and clinicians study DNA, classify cells, and investigate the mechanisms of disease. The team says that the device, which is small enough to fit in the palm of your hand, uses a beam of light emitted by a silicon photonics chip to manipulate particles millimetres away from the chip surface. They add that the light can penetrate the glass cover slips that protect samples used in biological experiments, enabling cells to remain in a sterile environment.
Traditional optical tweezers, which trap and manipulate particles using light, usually require bulky microscope setups, but chip-based optical tweezers could offer a more compact, mass manufacturable, broadly accessible, and high-throughput solution for optical manipulation in biological experiments.
“With silicon photonics, we can take this large, typically lab-scale system and integrate it onto a chip. This presents a great solution for biologists, since it provides them with optical trapping and tweezing functionality without the overhead of a complicated bulk-optical setup,” says Jelena Notaros, the Robert J. Shillman Career Development Professor in Electrical Engineering and Computer Science (EECS), and a member of the Research Laboratory of Electronics.
But so far, chip-based optical tweezers have only been capable of emitting light very close to the chip surface, so these prior devices could only capture particles a few microns off the chip surface. Biological specimens are typically held in sterile environments using glass cover slips that are about 150 microns thick, so the only way to manipulate them with such a chip is to take the cells out and place them on its surface. However, that leads to chip contamination. Every time a new experiment is done, the chip has to be thrown away and the cells need to be put onto a new chip.
According to the MIT researchers, their silicon photonics chip overcomes these challenges by emitting a beam of light that focuses about 5 mm above its surface. This way, they can capture and manipulate biological particles that remain inside a sterile cover slip, protecting both the chip and particles from contamination.
“This work opens up new possibilities for chip-based optical tweezers by enabling trapping and tweezing of cells at much larger distances than previously demonstrated. It’s exciting to think about the different applications that could be enabled by this technology,” says Notaros.
Manipulating light
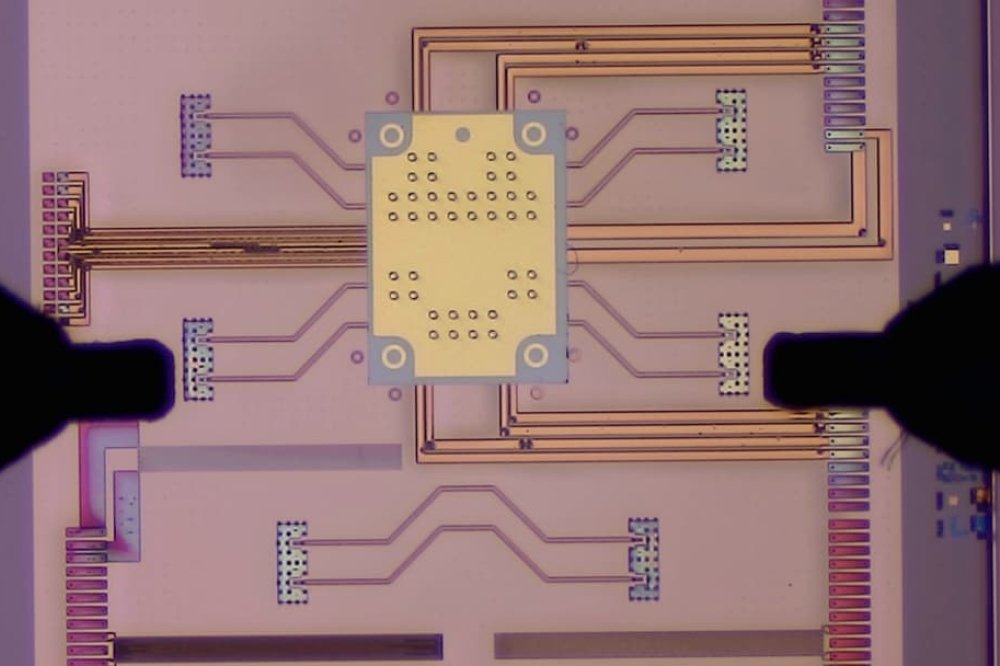
The researchers say they accomplished this using a system called an integrated optical phased array. This technology involves a series of microscale antennas fabricated on a chip using semiconductor manufacturing processes. By electronically controlling the optical signal emitted by each antenna, researchers can shape and steer the beam of light emitted by the chip.
Motivated by long-range applications like LiDAR, most prior integrated optical phased arrays weren’t designed to generate the tightly focused beams needed for optical tweezing. The MIT team discovered that, by creating specific phase patterns for each antenna, they could form an intensely focused beam of light, which can be used for optical trapping and tweezing millimetres from the chip’s surface.
“No one had created silicon-photonics-based optical tweezers capable of trapping microparticles over a millimetre-scale distance before. This is an improvement of several orders of magnitude higher compared to prior demonstrations,” says Notaros.
By varying the wavelength of the optical signal that powers the chip, the researchers could steer the focused beam over a range larger than a millimetre and with microscale accuracy. To test their device, the researchers started by trying to capture and manipulate tiny polystyrene spheres, before moving on to trapping and tweezing cancer cells.
“There were many unique challenges that came up in the process of applying silicon photonics to biophysics,” says Tal Sneh, lead author and EECS graduate student. The researchers had to determine how to track the motion of sample particles in a semi-automated fashion, ascertain the proper trap strength to hold the particles in place, and effectively postprocess data, for instance. In the end, they were able to show the first cell experiments with single-beam optical tweezers.
Building on these results, the team hopes to refine the system to enable an adjustable focal height for the beam of light. They also want to apply the device to different biological systems and use multiple trap sites at the same time to manipulate biological particles in more complex ways.
“This is a very creative and important paper in many ways,” says Ben Miller, Dean’s Professor of Dermatology and professor of biochemistry and biophysics at the University of Rochester, who was not involved with this work. “For one, given that silicon photonic chips can be made at low cost, it potentially democratises optical tweezing experiments. That may sound like something that only would be of interest to a few scientists, but in reality having these systems widely available will allow us to study fundamental problems in single-cell biophysics in ways previously only available to a few labs given the high cost and complexity of the instrumentation. I can also imagine many applications where one of these devices (or possibly an array of them) could be used to improve the sensitivity of disease diagnostic.”