Axithra raises €10m for biomedical application of photonics

Axithra, a new spin-off of imec and Ghent University, has announced a €10 million seed round, securing the first two years of R&D. Axithra develops a technology platform for therapeutic drug monitoring (TDM) to quickly and accurately measure drug concentrations in a patient’s blood.
The seed round led by imec.xpand and co-led by Kurma Partners amounted to €10 million, supported by an investor base including Qbic, Noshaq, White Fund, and Wallonie Entreprendre, as well as the companies Hamamatsu Photonics and Werfen Diagnostics.
For many drugs, the correct dosage is crucial to ensure the optimal effect. This is a constant focus in hospital units with seriously ill or debilitated patients, who often exhibit physiological changes over time, such as those in intensive care or oncology units. Insufficient doses mean a drug loses effectiveness, while excessive doses may cause toxic, potentially fatal, side effects. To address this challenge, Axithra will develop a therapeutic drug monitoring (TDM) platform based on optical technology. It aims at fast and accurate measurement of drug concentrations in blood, enabling timely adjustments of dosages as needed.
As a first application, the spin-off will deploy its technology to measure the concentration of beta-lactam antibiotics in a patient’s blood. This class of antibiotics is by far the most commonly used to treat or prevent bacterial infections and is administered to millions of intensive care patients each year. Axithra’s platform will ensure that treatments can be optimally tailored to the individual patient. Over time, other drug classes will be incorporated into the pipeline.
Prof. Jan De Waele, intensivist at Ghent University Hospital and president-elect of the European Society of Intensive Care Medicine, commented: “Given the large variation between patients in intensive care, this development will enable us to better treat our patients with severe infections, and protect them from possible harm. Because current solutions have long turnaround times, Axithra’s platform will help us to intervene more quickly, improving outcomes of severe infections and reducing the length of stay of patients in the intensive care unit, hereby decreasing costs.”
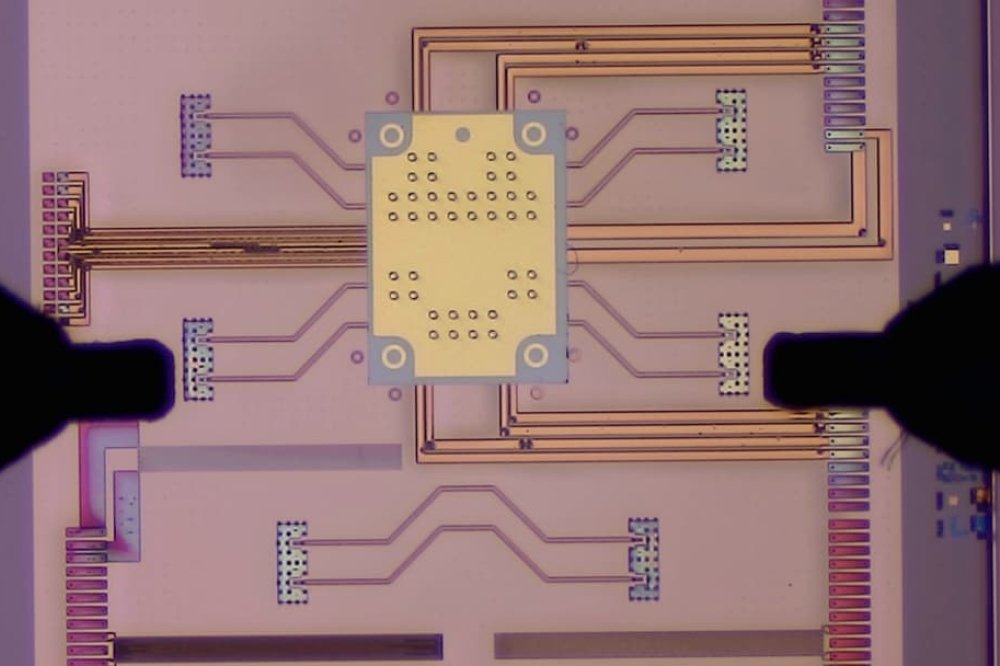
Axithra is an example of how processes developed for the semiconductor industry are now leveraged in the life sciences domain. Axithra combines imec’s semiconductor process knowledge with the photonics expertise of the Photonics Research Group, an associated imec lab at Ghent University. Prof. Roel Baets explained: “Our Raman-on-chip technology is the basis of Axithra’s solution. Raman spectroscopy is a commonly used technique for accurately identifying and quantifying molecules. Integration on a photonics chip makes this technique much more sensitive.”
Dr. Leander Van Neste, CEO of Axithra commented: “I am delighted with this broad, complementary investor base, including the regional and strategic support. I am convinced that together we can build out our TDM platform to be a true game-changer. Due to the simplicity and speed of our platform, we can customize medication for each individual patient, even with rapidly changing conditions, and in all sorts of environments, including outside the traditional hospital lab.”
Frank Bulens, partner at imec.xpand said: “Great to see such a broad investor support for this new spin-off from imec and UGent. This substantial seed round will allow the startup to achieve its prototype proof of concept milestone, a good basis for raising further financing to advance its product to the market.”
Alain Horvais, partner at Kurma Partners added: “We are confident that with a dynamic entrepreneurial team, Axithra will be able to provide physicians with a breakthrough TDM solution for a personalized and optimized patient treatment. Kurma Partners, through its specialized fund Kurma Diagnostics2, is pleased to support Leander and the Axithra team in this project.”