MedLumics Appoints new CEO
Spanish biophotonics start-up positions itself for commercialisation of optically guided cardiac surgery tool
MedLumics, a Spanish biophotonics company developing an optically guided cardiac ablation platform for the treatment of Atrial fibrillation (AF), has appointed James Greene as CEO and named Giovanni Leo to the board of directors.
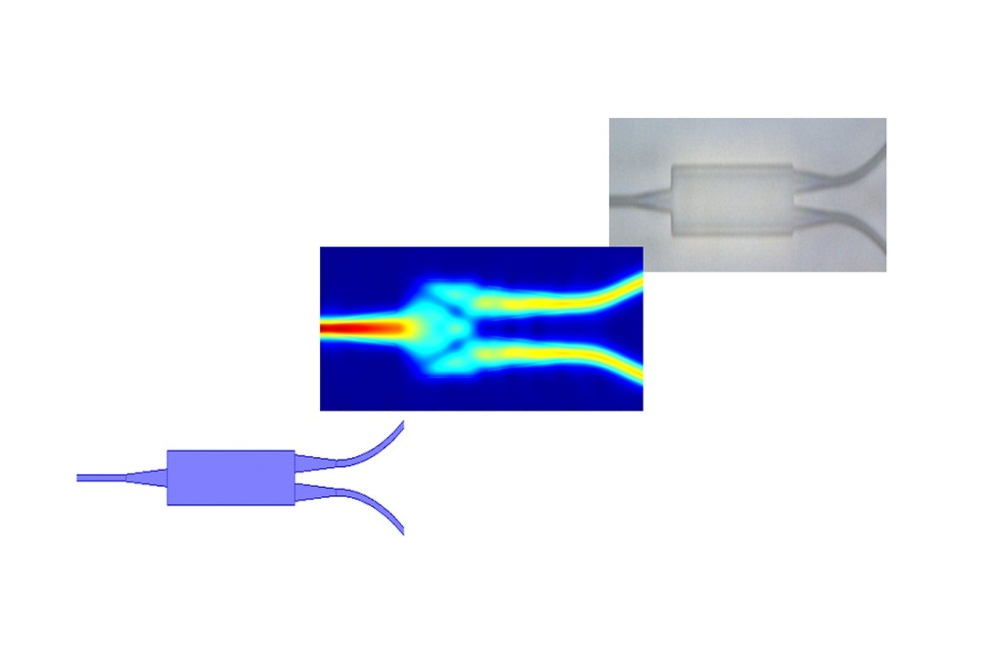
Founded in 2011, MedLumics has developed a proprietary integrated photonics platform that combines optical and electrical components in a miniature chip, enabling for the first time multi-view optical sensing for high-quality real-time images during cardiac ablation. The systems allow physicians to improve diagnostic and therapeutic procedures through a non-invasive optical evaluation of tissue.
The addition of two medical device industry veterans positions the company for its next phase of growth toward the commercialisation of its AblaView catheter, intended for the treatment of AF and other arrhythmias.
Eduardo Margallo Balbas and Jose Luis Rubio, MedLumics' Founders, continue to spearhead MedLumics' scientific innovation and product development as COO and CTO, respectively.
Greene, until recently a member of the MedLumics board of directors, brings 30 years of experience as an effective business leader in the medical device industry. He joins MedLumics from Seroba Lifesciences, a venture capital firm based in Dublin, where he was a partner.
In that role, Greene served as board director for the firm's medical device portfolio companies and mentored portfolio company management teams. Prior to Seroba, Greene founded and led cardiovascular device companies based in the US and Europe, including VERSO Technologies, APK Advanced Medical Technologies, APICA Cardiovascular, and MitralSolutions, for which he found a diversity of successful industrial acquirers.
Greene has also held leadership positions at large medical device companies, including Medtronic, Guidant Corporation, and Abbott Diagnostics.
"I am honoured to be chosen to lead MedLumics as CEO," said Greene. "MedLumics' innovative AblaView optically guided ablation technology enables clinicians to finally confirm in real time the creation of transmural scars without relying on a surrogate marker. The gravity of this innovation is the potential to reduce recurrence rates of AF, improve patient care, and provide a more cost-effective solution for the treatment of cardiac arrhythmias in today's value-based medicine environment. MedLumics has a stellar team of professionals focused on developing innovative product solutions that offer clinicians better options for treating patients who suffer from AF and other arrhythmias."
MedLumics also added new technical expertise to its leadership team with the appointment of Giovanni Leo to the board of directors. Before joining MedLumics' board, Leo served as vice president of research and development for St. Jude Medical.
He also previously co-founded and served as chief technology officer for Endosense, which invented the novel contact-force sensing catheter ablation technology for the treatment of cardiac arrhythmias and was acquired by St. Jude in 2013. Leo's extensive industry and engineering background will help guide MedLumics in its efforts to further advance optically guided minimally invasive instruments.
"There is a growing need for new technologies to further advance the catheter ablation treatment of arrhythmias, and MedLumics has an opportunity to deliver on this with its highly advanced optics technology," said Leo. "I look forward to lending my expertise as the company focuses on raising the bar in this field."
Olivier Litzka, MedLumics' chairman of the board, noted, "With Jim and Giovanni joining at the top management level, and with some further technical AF medtech experts having recently come to work at the company, MedLumics has nearly completed its leadership team. With such profound medtech company building and electrophysiology expertise along with the outstanding technology platform developed by the founders, MedLumics is in good shape to deliver on its technical and clinical milestones."
AF is the most common type of heart arrhythmia. An estimated 33.5 million people worldwide suffered from AF in 20101, and prevalence of the disease is expected to increase as the number of people over the age of 65 continues to expand2. Although many technologies have emerged over the last several years to improve the catheter ablation treatment of AF, the procedure remains highly complex and in part difficult to predict. Broad registries reveal recurrence rates of up to 60 percent at the one-year follow-up time point. The major widely accepted cause of recurrence after initially successful treatment is pulmonary vein reconnection due to ineffective lesion formation.
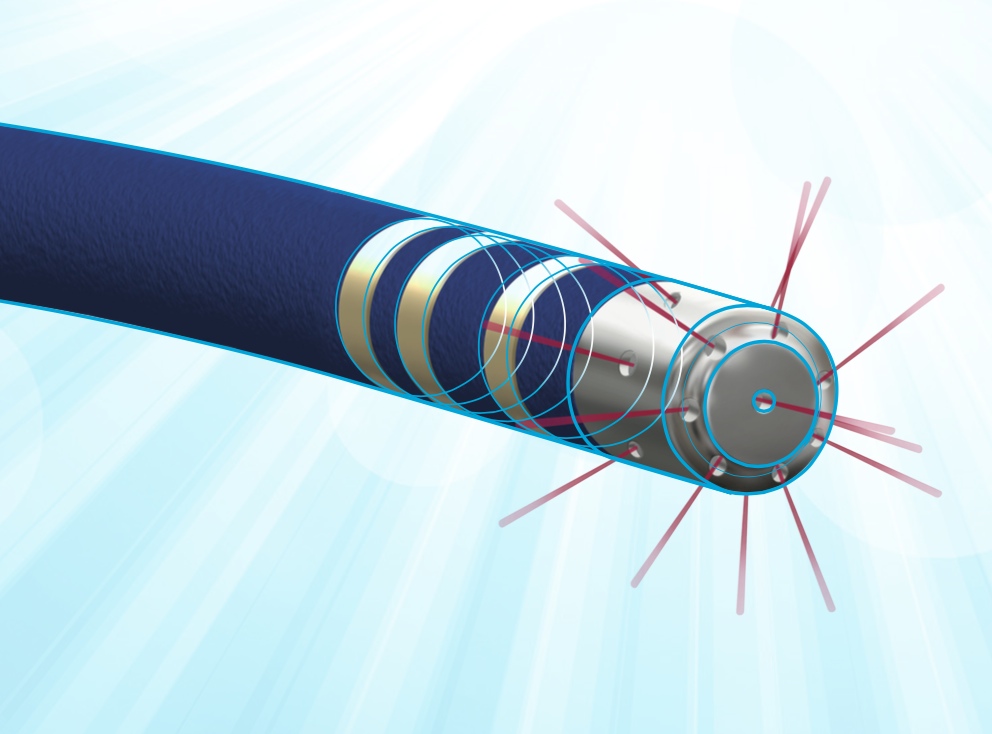
MedLumics' frontline product, AblaView, is a catheter-based radiofrequency (RF) ablation technology that optically guides catheter positioning and ablation treatment strategy using Optical Coherence Reflectometry (OCR).
AblaView's proprietary multibeam technology provides real-time, direct visual, confirmation of catheter contact, contact stability, and lesion transmurality during tissue ablation, reducing recurrence rates and reducing procedure technical complexity while increasing procedural safety. Although AF patients are expected to benefit most from this innovation, AblaView is a platform technology with multiple therapeutic application using RF ablation. The company is currently completing development of the AblaView Multibeam catheter and preparing for first in human clinical studies.
Earlier this year, MedLumics announced it raised €34.4 million in financing to help advance the product and clinical development of the company's AblaView catheter. The financing was the largest in the history of medtech in Spain and one of the largest in Europe in 2017.